Horizant® takes the bite out of nerve pain from PHN that remains after shingles
In a clinical study of people with Postherpetic Neuralgia (PHN), Horizant® delivered a clinical benefit in PHN pain scores (a ≥30-point improvement from baseline in more than half the patients)13
Review the data
Horizant® 600 mg BID significantly reduced PHN pain as early as Week 112*
- Reduction in pain score was 2x greater than placebo at Week 1 with Horizant® 600 mg BID12

*Secondary endpoint: This was a secondary endpoint of the study.13 These results should be interpreted within the context of the study design, which was not powered to detect a difference in this endpoint. Therefore, the ability to interpret the P value as significant is reduced.
Reduction of 24-hour average pain score during Week 1 up-titration vs placebo12
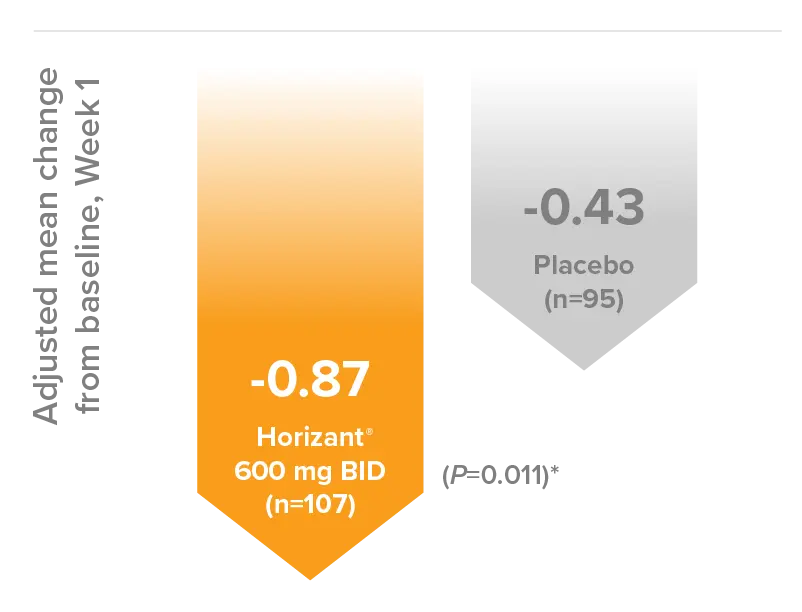
Reduction in PHN intensity (10-point scale) from baseline for Horizant® vs placebo (P=0.011)12*:
- Horizant® 600 mg BID (n=107): –0.87
- Placebo (n=95): –0.43
*Secondary endpoint: This was a secondary endpoint of the study.13 These results should be interpreted within the context of the study design, which was not powered to detect a difference in this endpoint. Therefore, the ability to interpret the P value as significant is reduced.
Patients on Horizant® reported significant improvement in 24-hour PHN nerve pain intensity over 13 weeks13
Reduction in 24-hour average pain intensity score by week13*,†

*Calculation based on adjusted mean change in 24-hour average pain intensity from baseline for the 600 mg group (2.47) and placebo group (1.66): 2.47 – 1.66 = 0.81; 0.81/1.66 = 0.4879 = 48.8%.13
†Horizant® 600 mg twice daily is the approved dose for PHN, and doses >600 mg twice daily increased side effects without additional benefit.1
- At Week 13, Horizant® 600 mg BID delivered a 49% reduction in mean 24-hour average pain intensity score vs placebo (–2.47 points vs –1.66 points for placebo; P=0.013)13
The efficacy and tolerability of Horizant® were evaluated in a randomized, double-blind, placebo-controlled study in adults with PHN. Patients (N=376) were required to have a baseline 24-hour average pain score of ≥4 on an 11-point pain intensity numerical rating scale, ranging from 0 (no pain) to 10 (worst possible pain). Patients were excluded if, over the past year, they had certain psychiatric comorbidities, a history of drug or alcohol abuse, hepatic or renal impairment (creatinine clearance <60 mL/min or hemodialysis), abnormal electrocardiogram, uncontrolled hypertension, or a seizure disorder requiring medication.1,13
The primary endpoint was change from baseline to end of maintenance treatment in mean 24-hour average pain intensity score. Additional endpoints included proportion of patients achieving a percent reduction in pain score from baseline and daytime/nighttime worst pain intensity.1,13
Horizant® significantly improved pain intensity score over the course of 14 weeks1,13
Horizant® 600 mg BID is the approved dose for PHN, and doses >600 mg BID increased side effects without additional therapeutic benefit1
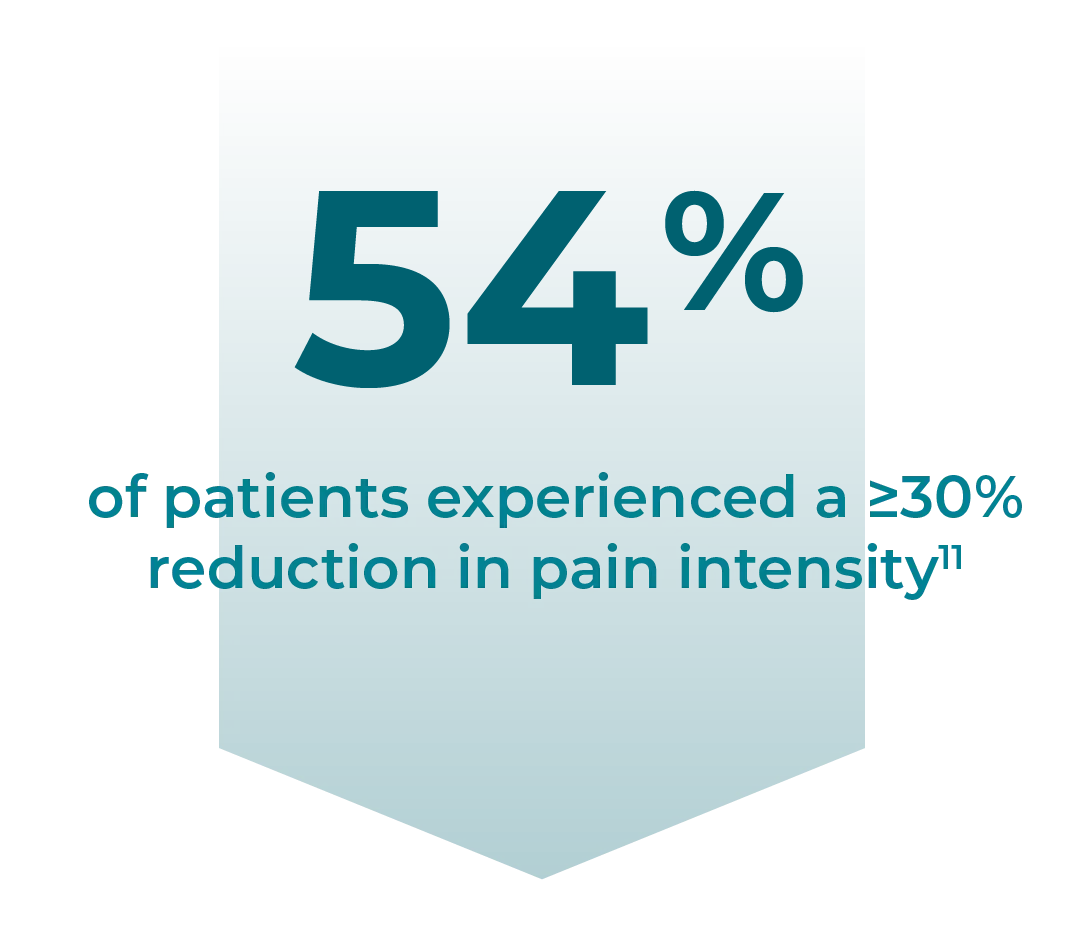
Improvement in pain intensity score from baseline to end of maintenance treatment1
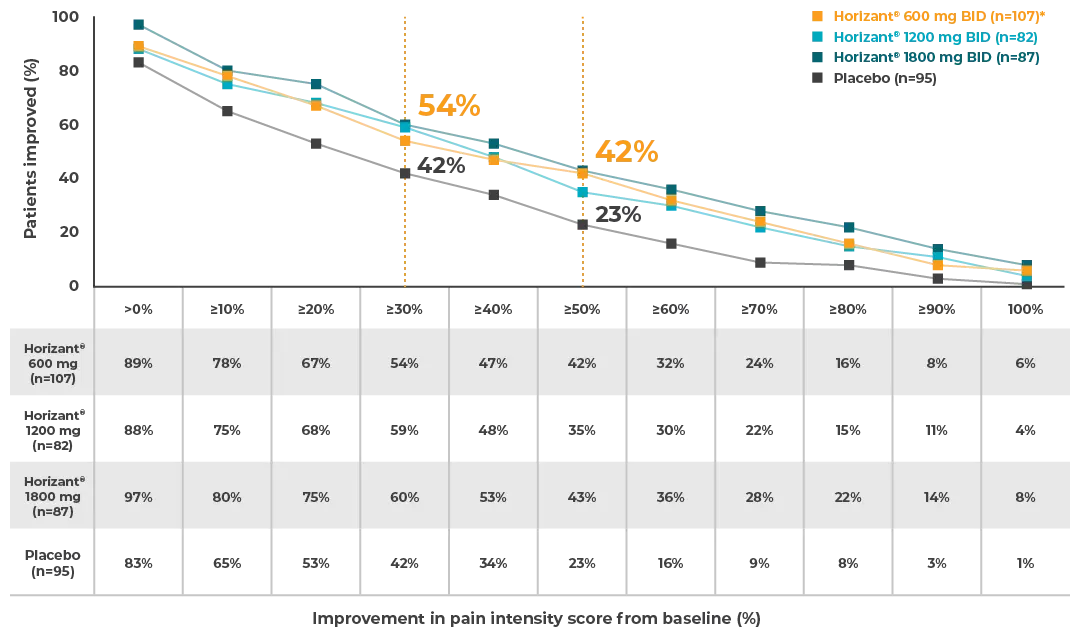
Improvement in pain intensity score from baseline to end of maintenance treatment1:
- Horizant® 600 mg BID (n=107)*:
– Percent patients who experienced:
>0% improvement in pain intensity score from baseline: 89%
≥10% improvement in pain intensity score from baseline: 78%
≥20% improvement in pain intensity score from baseline: 67%
≥30% improvement in pain intensity score from baseline: 54%
≥40% improvement in pain intensity score from baseline: 47%
≥50% improvement in pain intensity score from baseline: 42%
≥60% improvement in pain intensity score from baseline: 32%
≥70% improvement in pain intensity score from baseline: 24%
≥80% improvement in pain intensity score from baseline: 16%
≥90% improvement in pain intensity score from baseline: 8%
≥100% improvement in pain intensity score from baseline: 6%
*Horizant® 600 mg BID is the approved dose for PHN, and doses >600 mg BID increased side effects without additional therapeutic benefit.1
- Horizant® 1200 mg BID (n=82):
– Percent patients who experienced:
>0% improvement in pain intensity score from baseline: 88%
≥10% improvement in pain intensity score from baseline: 75%
≥20% improvement in pain intensity score from baseline: 68%
≥30% improvement in pain intensity score from baseline: 59%
≥40% improvement in pain intensity score from baseline: 48%
≥50% improvement in pain intensity score from baseline: 35%
≥60% improvement in pain intensity score from baseline: 30%
≥70% improvement in pain intensity score from baseline: 22%
≥80% improvement in pain intensity score from baseline: 15%
≥90% improvement in pain intensity score from baseline: 11%
≥100% improvement in pain intensity score from baseline: 4%
- Horizant® 1800 mg BID (n=87):
– Percent patients who experienced:
>0% improvement in pain intensity score from baseline: 97%
≥10% improvement in pain intensity score from baseline: 80%
≥20% improvement in pain intensity score from baseline: 75%
≥30% improvement in pain intensity score from baseline: 60%
≥40% improvement in pain intensity score from baseline: 53%
≥50% improvement in pain intensity score from baseline: 43%
≥60% improvement in pain intensity score from baseline: 36%
≥70% improvement in pain intensity score from baseline: 28%
≥80% improvement in pain intensity score from baseline: 22%
≥90% improvement in pain intensity score from baseline: 14%
≥100% improvement in pain intensity score from baseline: 8%
- Placebo (n=95):
– Percent patients who experienced:
>0% improvement in pain intensity score from baseline: 83%
≥10% improvement in pain intensity score from baseline: 65%
≥20% improvement in pain intensity score from baseline: 53%
≥30% improvement in pain intensity score from baseline: 42%
≥40% improvement in pain intensity score from baseline: 34%
≥50% improvement in pain intensity score from baseline: 23%
≥60% improvement in pain intensity score from baseline: 16%
≥70% improvement in pain intensity score from baseline: 9%
≥80% improvement in pain intensity score from baseline: 8%
≥90% improvement in pain intensity score from baseline: 3%
≥100% improvement in pain intensity score from baseline: 1%
*Horizant® 600 mg BID is the approved dose for PHN, and doses >600 mg BID increased side effects without additional therapeutic benefit.1
Patients with PHN discontinued placebo at higher rates than Horizant®1*
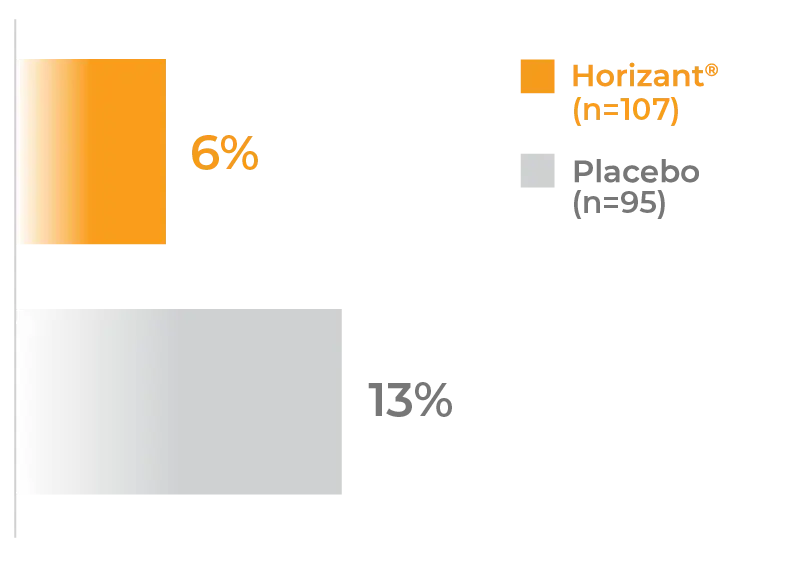
- 6% of patients taking Horizant® 1200 mg (n=107) discontinued due to adverse reactions vs 13% for placebo (n=95)1
*From the Horizant® 600 mg BID study arm of the PHN pivotal trial (n=107). Horizant® 600 mg BID is the approved dose for PHN, and doses >600 mg BID increased side effects without additional therapeutic benefit.1
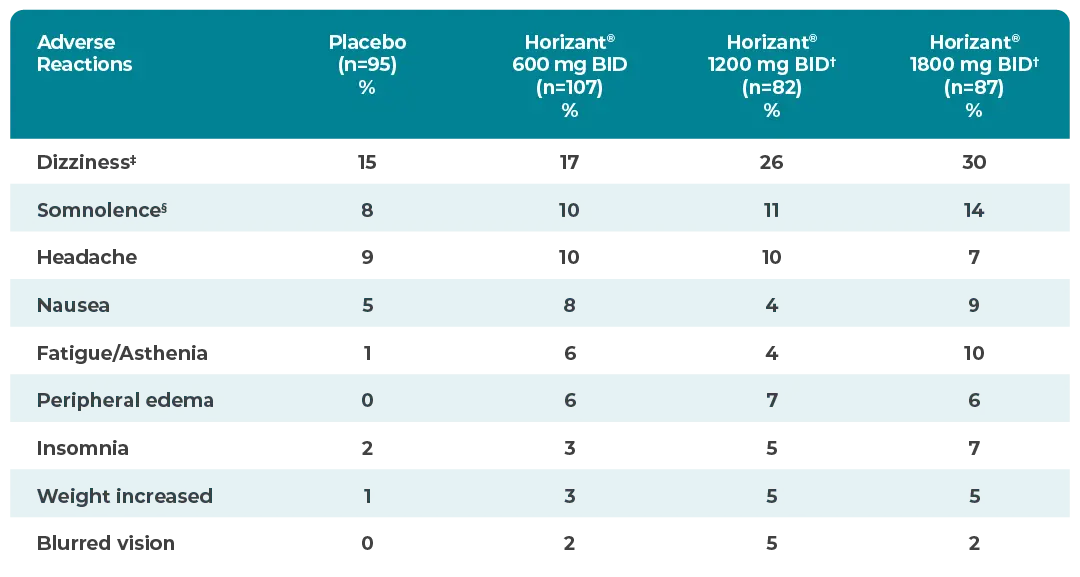
Horizant® was well tolerated in the PHN pivotal trial1
*Treatment-emergent adverse reactions that occurred in ≥2% of patients with PHN treated with Horizant® 600 mg twice daily and numerically greater than placebo in a placebo-controlled, 12-week clinical trial.1
†Horizant® 600 mg twice daily is the approved dose for PHN, and doses >600 mg twice daily increased side effects without additional benefit.1
‡In patients treated with Horizant® 600 mg twice daily who reported dizziness (17%), symptoms persisted during treatment in about 6%.1
§In patients treated with Horizant® 600 mg twice daily who reported somnolence (10%), symptoms persisted during treatment in about 27%. In remaining patients, somnolence resolved within 4 to 5 weeks.1
Most common adverse reactions vs placebo (≥2% of patients on Horizant®)1*
| Adverse Reactions | Placebo (n=95)
% |
Horizant® 600 mg BID (n=107)
% |
Horizant 1200 mg BID*
% |
Horizant® 1800 mg BID† (n=87)
% |
| Dizziness‡ | 15 | 17 | 26 | 30 |
| Somnolence§ | 8 | 10 | 11 | 14 |
| Headache | 9 | 10 | 10 | 7 |
| Nausea | 5 | 8 | 4 | 9 |
| Fatigue/Asthenia | 1 | 6 | 4 | 10 |
| Peripheral edema | 0 | 6 | 7 | 6 |
| Insomnia | 2 | 3 | 5 | 7 |
| Weight increased | 1 | 3 | 5 | 5 |
| Blurred Vision | 0 | 2 | 5 | 2 |
*Treatment-emergent adverse reactions that occurred in ≥2% of patients with PHN treated with Horizant® 600 mg twice daily and numerically greater than placebo in a placebo-controlled, 12-week clinical trial.1
†Horizant® 600 mg twice daily is the approved dose for PHN, and doses >600 mg twice daily increased side effects without additional benefit.1
‡In patients treated with Horizant® 600 mg twice daily who reported dizziness (17%), symptoms persisted during treatment in about 6%.1
§In patients treated with Horizant® 600 mg twice daily who reported somnolence (10%), symptoms persisted during treatment in about 27%. In remaining patients, somnolence resolved within 4 to 5 weeks.1
BID=twice daily.
Important Safety Information for HORIZANT® (gabapentin enacarbil) Extended-Release Tablets
INDICATIONS:
HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients who are required to sleep during the daytime and remain awake at night.
HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the management of postherpetic neuralgia (PHN) in adults.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Effects on Driving
HORIZANT may cause significant driving impairment. The duration of driving impairment after starting therapy is unknown. Patients should not drive until they have enough experience on HORIZANT to know if it impairs their driving. Patients’ ability to assess their driving competence and degree of somnolence caused by HORIZANT can be imperfect.
Somnolence/Sedation and Dizziness
HORIZANT causes somnolence/sedation and dizziness. Patients should not drive or operate other complex machinery until they have enough experience on HORIZANT to know if it impairs their ability to perform these tasks.
Lack of Interchangeability with Gabapentin
HORIZANT is not interchangeable with other gabapentin products because of differing pharmacokinetic profiles. The same dose of HORIZANT results in different plasma concentrations of gabapentin relative to other gabapentin products. The safety and effectiveness of HORIZANT in patients with epilepsy have not been studied.
Suicidal Behavior and Ideation
HORIZANT is a prodrug of gabapentin, an antiepileptic drug (AED). AEDs increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. As a prodrug of gabapentin, HORIZANT also increases this risk. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Anyone considering prescribing HORIZANT must balance the risk of suicidal thoughts or behavior with the risk of untreated illness.
Patients, caregivers, and families should be informed that HORIZANT increases the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts of self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Respiratory Depression
There is evidence from case reports, human studies, and animal studies associating gabapentin with serious, life-threatening, or fatal respiratory depression when co-administered with central nervous system (CNS) depressants, including opioids, or in the setting of underlying respiratory impairment. When the decision is made to co-prescribe HORIZANT with another CNS depressant, particularly an opioid, or to prescribe HORIZANT to patients with underlying respiratory impairment, monitor patients for symptoms of respiratory depression and sedation, and consider initiating HORIZANT at a low dose. The management of respiratory depression may include close observation, supportive measures, and reduction or withdrawal of CNS depressants (including HORIZANT).
Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan hypersensitivity, has been reported in patients taking antiepileptic drugs, including gabapentin. HORIZANT is a prodrug of gabapentin. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, and/or lymphadenopathy, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis sometimes resembling an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its expression, other organ systems not noted here may be involved.
It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. HORIZANT should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
Discontinuation of HORIZANT
When discontinuing HORIZANT, patients with RLS receiving 600 mg or less once daily can discontinue the drug without tapering. If the recommended dose is exceeded, the dose should be reduced to 600 mg daily for 1 week prior to discontinuation to minimize the potential of withdrawal seizure.
In patients with PHN receiving HORIZANT twice daily, the dose should be reduced to once daily for 1 week prior to discontinuation to minimize the potential of withdrawal seizure.
Tumorigenic Potential
In an oral carcinogenicity study, gabapentin enacarbil increased the incidence of pancreatic acinar cell adenoma and carcinoma in male and female rats. The clinical significance of this finding is unknown.
ADVERSE REACTIONS
The most common adverse reactions for patients with RLS (incidence >10% and at least 2 times the rate of placebo) were somnolence/sedation and dizziness.
The most common adverse reactions for patients with PHN (incidence >10% and greater than placebo) were dizziness, somnolence, and headache.
DRUG INTERACTIONS
Gabapentin enacarbil is released faster from HORIZANT Extended-Release tablets in the presence of
alcohol. Consumption of alcohol is not recommended when taking HORIZANT.
HORIZANT taken in conjunction with morphine causes increased somnolence/sedation, dizziness, and
nausea.
USE IN SPECIAL POPULATIONS
Pregnancy and Lactation
There are no adequate data on the developmental risk associated with the use of HORIZANT in pregnant women. In nonclinical studies in rats and rabbits, administration of gabapentin enacarbil was developmentally toxic when administered to pregnant animals at doses and gabapentin exposures greater than those used clinically.
It is not known whether gabapentin derived from HORIZANT is secreted in human milk; however, gabapentin is secreted into human milk following oral administration of other gabapentin products. There are no data on the effects of gabapentin on the breastfed infant or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for HORIZANT and any potential adverse effects on the breastfed infant from HORIZANT or from the underlying maternal condition.
Pediatric Use
Safety and effectiveness of HORIZANT in pediatric patients have not been studied.
Geriatric Use
Clinical trials of HORIZANT for the treatment of RLS did not include a sufficient number of patients 65 years and older to determine whether they respond differently from younger individuals. Because elderly patients are more likely to have decreased renal function, the frequency of dosing may need to be adjusted based on calculated creatinine clearance in these patients.
Renal Impairment
Gabapentin is known to be almost exclusively excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. The dose of Horizant should be adjusted in patients with renal impairment based upon creatinine clearance. HORIZANT is not recommended for treatment of RLS in patients receiving hemodialysis.
For additional safety information, please see the complete Prescribing Information for HORIZANT.
To report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449, or FDA at 1-800-FDA-1088 or www.fda.gov/MedWatch.
References: 1. Horizant [package insert] Woburn, MA: Azurity Pharmaceuticals, Inc.; 2022 2. Food and Drug Administration. Orange Book: approved drug products with therapeutic equivalence evaluations [gabapentin enacarbil]. Accessed November 17, 2022. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm 3. Cundy KC, Sastry S, Luo W, et al. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol. 2008;48(12):1378-1388. 4. Gralise [prescribing information]. Morristown, NJ: Almatica Pharma LLC. 5. Neurontin [prescribing information]. New York, NY: Parke-Davis Division of Pfizer Inc. 6. Lal R, Ellenbogen A, Gidal B. Interindividual variability in the bioavailability of gabapentin enacarbil extended release in healthy adults: an analysis of data from 6 phase I studies. Ther Drug Monit. 2022;44(3):448-454. 7. Swearingen D, Aronoff GM, Ciric S, et al. Pharmacokinetics of immediate release, extended release, and gastric retentive gabapentin formulations in healthy adults. Int J Clin Pharmacol Ther. 2018;56(5):231-238. 8. Yang JY, Lee WI, Shin WK, et al. Administration of four different doses of gabapentin reduces awakening from breakthrough pain and adverse effects in outpatients with neuropathic pain during the initial titration. Korean J Anesthesiol. 2013;65(1):48-54. 9. Lee DO, Ziman RB, Perkins AT, et al; XP053 Study Group. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med. 2011;7(3):282-292. 10. Walters AS, LeBrocq C, Dhar A, et al; International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4(2):121-132. 11. Silber MH, Buchfuhrer MJ, Earley CJ, et al. The management of restless legs syndrome: an updated algorithm. Mayo Clin Proc. 2021;96(7):1921-1937. 12. Data on file. Arbor Pharmaceuticals, LLC. 13. Zhang L, Rainka M, Freeman R, et al. A randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of gabapentin enacarbil in subjects with neuropathic pain associated with postherpetic neuralgia (PXN110748). J Pain. 2013;14(6):590-603.
Are you a US Healthcare Professional?
This portion of the website is intended for US healthcare professionals.
Go to patient site Continue